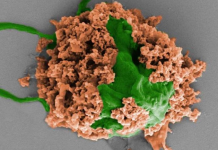
Prostate Cancer: The FDA, the US regulatory agency, on January 10 approved a genetic risk test for prostate cancer by the biotechnology company 23andMe. The product does not require a medical prescription and, according to the company, is more than 99% accurate. After taking the test, the consumer will have access to an educational module that will help him to interpret the results.
Anne Wojcicki, executive director of 23andMe, says the product allows the user to know if they are at risk of developing prostate cancer and, together with doctors, take preventive measures.
Although the FDA has already authorized the product, 23andMe has yet to release a release date.
This is the third approval for genetic cancer risk testing that the company has obtained from the FDA. The other two (breast and colorectal cancer) are already part of the agency’s risk assessment system.
However, the test does not include all genetic mutations at risk for prostate cancer, considering only the G84E variant, which is more common in people of European descent.
The product also does not consider lifestyle factors that can increase the chances of developing the disease, such as smoking.